FDA Warns Against Use of Counterfeit Ozempic (Semaglutide) in U.S. Drug Supply
FDA Warns Against Use of Counterfeit Ozempic. The FDA warns consumers and healthcare providers about counterfeit Ozempic (semaglutide) found in the U.S. drug supply chain. Learn how to identify fake products and protect your health.

On April 3, 2025, Novo Nordisk notified the FDA that several hundred units of counterfeit Ozempic (semaglutide) injection 1mg had entered the U.S. drug supply chain. These counterfeit products were distributed outside of Novo Nordisk’s authorized supply network. The FDA seized the identified counterfeit units on April 9, 2025.
The FDA urges patients, wholesalers, pharmacies, and healthcare providers to inspect any Ozempic products they have. Do not use, sell, or distribute products labeled with lot number PAR0362 and serial numbers beginning with 51746517, as shown in the image below.
So far, six adverse events have been reported to Novo Nordisk related to this lot, but none appear linked to the counterfeit products.
The FDA and Novo Nordisk are currently testing the seized items to determine their identity, quality, and safety. The investigation remains ongoing as the agency works to safeguard the U.S. drug supply.
The FDA is continuing its investigation into counterfeit Ozempic (semaglutide) injection 1 mg that has entered the legitimate U.S. drug supply chain. To date, the agency has seized thousands of counterfeit units.
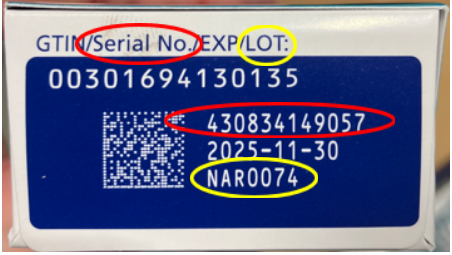
The FDA urges wholesalers, pharmacies, healthcare providers, and patients to examine their Ozempic products carefully. Do not use, sell, or distribute any product labeled with lot number NAR0074 and serial number 430834149057, as shown below.
Some counterfeit units may still be in circulation and available for purchase.
The FDA and Novo Nordisk, the manufacturer of Ozempic, are currently testing the seized counterfeit products. At this time, there is no confirmed information about the identity, quality, or safety of the drugs involved.
Initial analysis has confirmed that the needles included with the counterfeit products are also fake. As a result, sterility cannot be guaranteed, increasing the risk of infection for anyone who uses them. Other confirmed counterfeit components include the pen label, carton, and both patient and healthcare provider information inserts.
The FDA is aware of five adverse events associated with this lot. None were serious, and all reported symptoms—nausea, vomiting, diarrhea, abdominal pain, and constipation—align with known side effects of authentic Ozempic.
To ensure safety, the FDA strongly recommends:
-
Retail pharmacies should purchase Ozempic only through authorized Novo Nordisk distributors and carefully review lot numbers, serial numbers, and product packaging for authenticity.
-
Patients should obtain Ozempic only with a valid prescription from a state-licensed pharmacy and inspect the product for any signs of tampering or counterfeiting before use.
The FDA takes reports of counterfeit drugs seriously and is working closely with other federal agencies and the private sector to protect the integrity of the U.S. drug supply. The investigation is ongoing, and the agency is coordinating with Novo Nordisk to identify, investigate, and remove additional suspected counterfeit semaglutide products from the market.
Reporting Adverse Events and Counterfeit Products
Healthcare professionals and consumers should report any side effects or suspected counterfeit products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
-
Submit reports online: MedWatch Online Voluntary Reporting Form
-
Or download the form and fax it to 1-800-FDA-0178.
Suspected criminal activity, including the sale of counterfeit or tampered medications (especially online), should be reported to the FDA via your local consumer complaint coordinator or directly on the FDA website.
For questions or concerns, patients and retailers can contact Novo Nordisk Customer Care at 1-800-727-6500, Monday–Friday, 8:30 a.m. to 6 p.m. ET.
FDA Seizes Thousands of Counterfeit Ozempic Pens in Ongoing Investigation — Here’s What You Need to Know
The U.S. Food and Drug Administration (FDA) is continuing its investigation into counterfeit Ozempic (semaglutide) injection 1 mg that has entered the legitimate U.S. drug supply chain. To date, the agency has seized thousands of counterfeit units and is urging healthcare providers, pharmacists, and patients to remain vigilant.
Ozempic, manufactured by Novo Nordisk, is a glucagon-like peptide-1 (GLP-1) receptor agonist. It’s approved by the FDA to improve glycemic control in adults with Type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular events, such as heart attack and stroke, in adults with both Type 2 diabetes and established cardiovascular disease.
Why Ozempic is in High Demand
While Ozempic is FDA-approved for Type 2 diabetes and heart health, it has also become popular off-label for weight loss—a use not originally approved but widely adopted. This off-label use, combined with its soaring popularity on social media, has contributed to significant demand and global shortages, creating opportunities for counterfeiters.
The retail price of Ozempic is approximately $998 for a month’s supply (four once-weekly injections), making it a lucrative target for counterfeiting.
Novo Nordisk also markets Wegovy, a higher-dose version of semaglutide that is specifically approved for weight loss. In 2024, Novo Nordisk reported $17 billion in sales from Ozempic and an additional $8 billion from Wegovy, reflecting the explosive growth of the GLP-1 drug category.
Competing in this space is Eli Lilly, with its own GLP-1-based drugs: Mounjaro (for Type 2 diabetes) and Zepbound (for weight loss). Both companies have seen massive commercial success as public interest in GLP-1 medications continues to rise.
What the FDA Recommends
To protect yourself and others from the risks of counterfeit medication, the FDA offers the following guidance:
-
Pharmacies and Wholesalers: Only purchase Ozempic through authorized Novo Nordisk distributors. Carefully check product packaging, lot numbers, and serial numbers against FDA alerts.
-
Patients: Always get Ozempic from a licensed U.S. pharmacy with a valid prescription. Before using, inspect the product for any irregularities or signs of tampering.
-
Healthcare Providers: Advise patients on how to spot counterfeit products and report anything suspicious.
The FDA and Novo Nordisk continue to test the seized counterfeit products to determine their contents and safety. The investigation is ongoing, and the agency is actively working to remove any additional counterfeit semaglutide products from the U.S. market.
Report Counterfeits or Side Effects
Anyone experiencing adverse reactions or suspecting counterfeit medication should report to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
-
Submit online: MedWatch Online Reporting Form
-
Or fax the completed form to 1-800-FDA-0178.
Suspected counterfeit drug activity, including online sales, can also be reported to the FDA through your local FDA consumer complaint coordinator or on the FDA website.